Hidden chemistry of Earth’s core revealed by how it froze
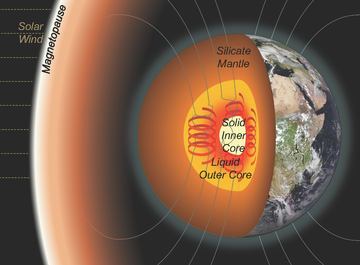
Cartoon of the Earth with cutaway showing the mantle and inner and outer core. Magnetic field lines produced by the geodynamo extend into space and interact with the solar wind. Image credit: Dr Alfred Wilson
Earth’s inner core, the iron-rich mass at the centre of our planet, is slowly growing as the surrounding molten outer core cools and freezes. But when did this freezing begin? This is a question that scientists have debated for decades, with some arguing for an ancient inner core (with freezing beginning more than two billion years ago) and others suggesting a much younger age (less than half a billion years). This is, in part, due to the lack of knowledge that researchers have about the exact chemistry of the core. Without this information, its physical properties (and therefore how it has evolved) remain enigmatic.
And as it turns out, identifying when the inner core formed isn’t just a matter of determining when the core cooled to its freezing point. Like water droplets in clouds, which can cool to -30°C before forming hail, molten iron must be supercooled (cooled to below its melting point) before it can freeze. Indeed, previous calculations have suggested that 800-1000 degrees of supercooling would be needed to initiate freezing of the core if it were made of pure iron.
However, if the core is supercooled to this degree, simulations have shown that the inner core would grow massively, and the Earth’s magnetic field would fail. But seismic observations of the inner core, and the existence of ancient, magnetised rocks, suggest that neither of these outcomes occurred during our planet’s history. Instead, scientists believe that in the past, the core could have cooled to no more than about 250°C below its melting point.
So how is it possible that there was such limited supercooling of the core, and yet the inner core exists as a solid layer today?
To explore this question, a research team from the University of Leeds, University of Oxford, and University College London, looked at how the presence of other elements, specifically silicon, sulphur, oxygen, and carbon, might affect the freezing process.
“Each of these elements exist in the overlying mantle and could therefore have been dissolved into the core during Earth’s history. As a result, these could explain why we have a solid inner core with relatively little supercooling at this depth. The presence of one or more of these elements could also rationalise why the core is less dense than pure iron, a key observation from seismology.”
- Professor Andrew Walker (Oxford Earth Sciences), study co-author
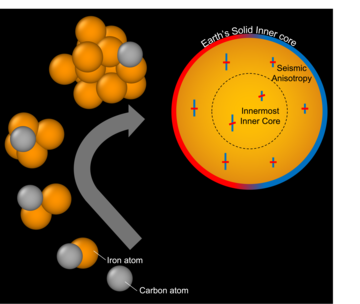
The authors used atomic scale simulations to identify solids nucleating in supercooled liquids. They found that carbon atoms can accelerate this process, perhaps enough to trigger the freezing of Earth's solid inner core. Image credit: Dr Alfred Wilson
Using atomic-scale computer simulations of around 100,000 atoms at supercooled temperatures and pressures equivalent to those in the inner core, the research team tracked how often small crystal-like clusters of atoms formed from a liquid. These “nucleation” events are the first steps toward freezing, but below a critical size, clusters are unstable. As a result, most of these clusters quickly remelt and only very rarely do they grow large enough to survive and initial large-scale freezing. By watching how often small clusters form, the researchers were able to estimate how often larger, critically sized ones might appear.
What they found was surprising: silicon and sulphur, elements often assumed to be present in the core, actually slow down the freezing process. In other words, more supercooling would be needed to start forming the inner core if these elements were abundant in the core. On the other hand, they found that carbon helped to accelerate freezing in the simulation.
In the study, the researchers tested how much supercooling would be required to freeze the inner core if 2.4 % of the core’s mass were made of carbon. The result: about 420°C, still too much to match observations, but the closest result to viability yet. But when they extrapolated their results to a case where 3.8 % of the core’s mass is carbon, the required supercooling dropped to 266°C. This is the only known composition that could explain both the nucleation and observed size of the inner core.
This result indicates that carbon may be more abundant in Earth’s core than previously thought, and that without this element, the formation of a solid inner core may never have happened. The experiments also show that inner core freezing was possible with just the right chemistry, and unlike water when it forms hail, it did so without “nucleation seeds”, tiny particles which help to initiate freezing. This is vital, because when tested in previous simulations, all of the candidates for nucleation seeds in the core have melted or dissolved.
“It is exciting to see how atomic scale processes control the fundamental structure and dynamics of our planet. By studying how Earth’s inner core formed, we are not just learning about our planet’s past. We’re getting a rare glimpse into the chemistry of a region we can never hope to reach directly and learning about how it could change in the future.”
- Alfred Wilson (University of Leeds), lead author of the study
The study ‘Constraining Earth’s core composition from inner core nucleation’ is available to read in the journal Nature Communications at https://doi.org/10.1038/s41467-025-62841-4. The work was funded by the Natural Environment Research Council (NERC).




